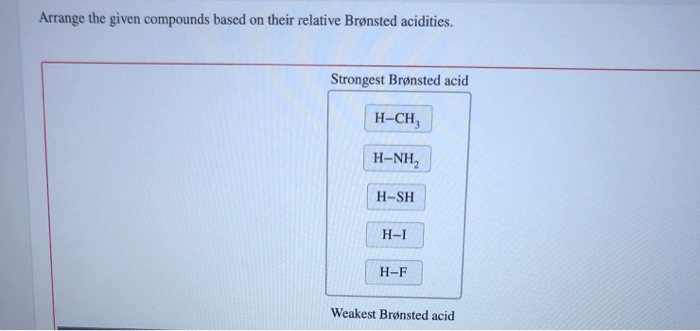
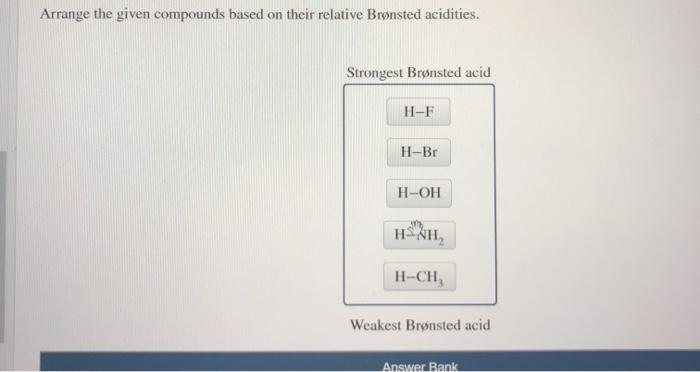
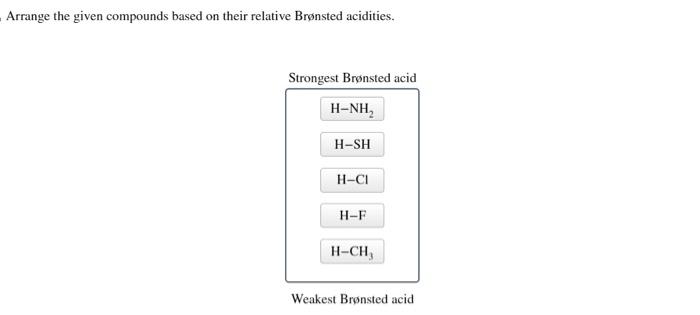
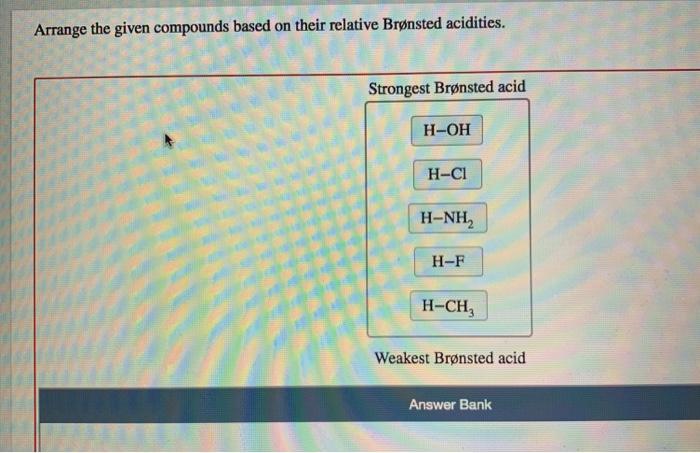
Arrange the given compounds based on their relative brønsted acidities. – The concept of Brønsted acidity, a cornerstone in chemistry, takes center stage as we delve into the intricacies of comparing the relative strengths of various compounds. Understanding Brønsted acidity is not merely an academic pursuit but holds immense practical significance, with applications spanning diverse fields from chemistry and biology to medicine.
As we embark on this exploration, we will unveil the factors that govern Brønsted acidity and develop a comprehensive understanding of how to arrange compounds based on their relative strengths.
To set the stage, we define Brønsted acidity and establish its relationship with the strength of the conjugate base. We will examine specific examples of strong and weak Brønsted acids, providing a solid foundation for our subsequent analysis.
Brønsted Acidities
A Brønsted acid is a substance that donates a proton (H+) to a base. The strength of a Brønsted acid is determined by the stability of its conjugate base. A strong acid has a weak conjugate base, while a weak acid has a strong conjugate base.
The following are some examples of strong and weak Brønsted acids:
- Strong acids: HCl, H2SO4, HNO3
- Weak acids: CH3COOH, H2CO3, NH4+
Factors Affecting Brønsted Acidity
The following factors affect the Brønsted acidity of a compound:
- Electronegativity of the atom bonded to hydrogen
- Size of the atom bonded to hydrogen
- Resonance stabilization of the conjugate base
The more electronegative the atom bonded to hydrogen, the stronger the Brønsted acid. This is because the more electronegative the atom, the more it will pull electrons away from hydrogen, making it more likely to donate a proton.
The larger the atom bonded to hydrogen, the weaker the Brønsted acid. This is because the larger the atom, the more it will shield the hydrogen from the electronegative atom, making it less likely to donate a proton.
Resonance stabilization of the conjugate base can increase the strength of a Brønsted acid. This is because resonance stabilization can spread out the negative charge of the conjugate base, making it more stable and less likely to accept a proton.
Relative Brønsted Acidity of Compounds, Arrange the given compounds based on their relative brønsted acidities.
The relative Brønsted acidity of compounds can be compared by looking at the pKa values of their conjugate bases. The lower the pKa value, the stronger the acid.
The following table shows the pKa values of some common acids:
| Acid | pKa |
|---|---|
| HCl | |
| H2SO4 | |
| HNO3 | |
| CH3COOH | |
| H2CO3 | |
| NH4+ |
As can be seen from the table, HCl is the strongest acid, followed by H2SO4, HNO3, CH3COOH, H2CO3, and NH4+.
Applications of Brønsted Acidity
Brønsted acidity is used in a wide variety of applications, including:
- Chemistry: Brønsted acids are used as catalysts in many chemical reactions. They are also used to protonate organic molecules, which can change their reactivity.
- Biology: Brønsted acids are involved in many biological processes, such as digestion, respiration, and photosynthesis.
- Medicine: Brønsted acids are used in a variety of medical applications, such as treating ulcers, heartburn, and cancer.
Commonly Asked Questions: Arrange The Given Compounds Based On Their Relative Brønsted Acidities.
What is the significance of Brønsted acidity?
Brønsted acidity is a fundamental property that governs the behavior of acids and bases in chemical reactions. It plays a crucial role in determining the strength of acids, the reactivity of bases, and the equilibrium positions of acid-base reactions.
How do we determine the relative acidity of different compounds?
The relative acidity of compounds can be determined by comparing the strength of their conjugate bases. A stronger acid will have a weaker conjugate base, and vice versa. Factors such as electronegativity, resonance stabilization, and inductive effects influence the strength of conjugate bases and, consequently, the relative acidity of compounds.
What are some practical applications of Brønsted acidity?
Brønsted acidity has numerous practical applications in various fields, including chemistry, biology, and medicine. In chemistry, it is used to optimize chemical reactions, design new materials, and understand reaction mechanisms. In biology, it plays a crucial role in enzyme catalysis, protein function, and DNA replication.
In medicine, it is essential for understanding drug interactions, designing new therapies, and treating diseases.